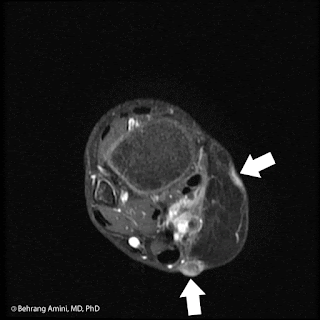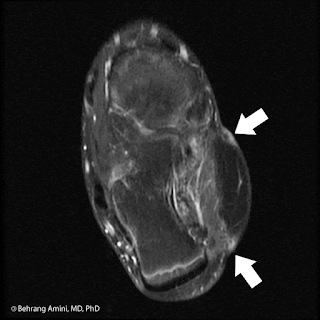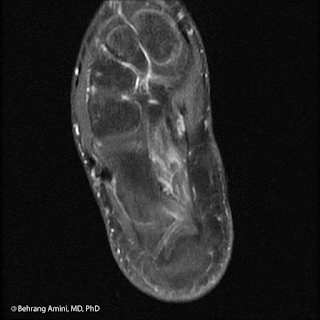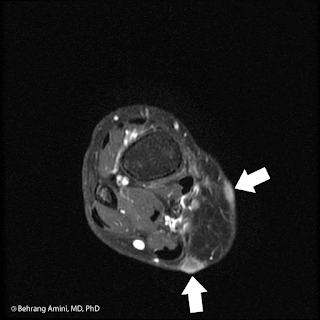
Serial contrast-enhanced axial images of the forearm in a patient with recurrent myxofibrosarcoma (MFS). Arrows indicate slowly enlarging, tail-like recurrence along the superficial fascia in the resection bed.
Myxofibrosarcoma (MFS, previously myxoid malignant fibrous histiocytoma) is an intermediate-grade soft-tissue sarcoma with fibroblastic and myxoid components. Patients are typically in the sixth to eighth decades of life.
These lesions can range from round and well-defined (like most soft tissue sarcomas) to predominantly infiltrating, with tails extending along fascial planes. They have high T2 signal intensity related to their myxoid content and heterogeneous enhancement that can be feather-like (typically seen with myxoid neoplasms).
Despite best efforts at acquiring negative margins (made difficult by infiltrating tails), MFS has a propensity for repeated local recurrence, with rates of up to 79%. Recurrence can be tricky to detect. As seen in the image above, tail-like recurrence can be easily dismissed as being related to inflammation or trauma. Our group has found no association between the appearance of MFS at presentation (well-defined vs. tail-like) and the pattern of recurrence, so it's important to watch out for tails regardless of the initial tumor presentation.
In summary, the key to early detection of recurrent MFS is
- Know that you're dealing with MFS (look at the path report, and know that some pathologists may still call these "myxoid MFH").
- Be aware of propensity for tail-like recurrence.
- Make sure to compare to multiple studies dating back to post-operative baseline to increase sensitivity for detection of these slowly growing lesions.