|
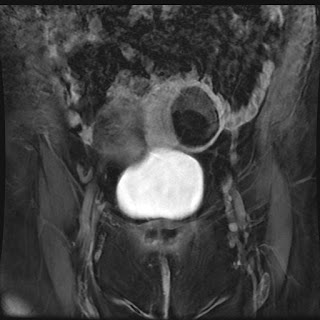
| Thick slab MRCP image showing massive dilatation of the main pancreatic duct |
- Wide variety of pathology both benign and malignant
- Imaging findings and demographics are the key to diagnosis
Cystic Pancreatic Neoplasms (Four major categories)
- Serous cystadenoma: Benign (very low malignant potential)
- Mucinous cystic neoplasm (MCN): Premalignant or malignant
- Intraductal papillary mucinous neoplasm (IPMN): Malignant potential (Main Duct >> Branch Duct)
- Unusual cystic neoplasms:
- Solid pseudopapillary neoplasm (SPN): Low grade malignancy
- Cystic forms of more common neoplasms (neuroendocrine)
Nonneoplastic Pancreatic Cysts
- Pseudocyst
- Retention cyst
- Lymphoepithelial cyst
- Localized ductectasia
Major Imaging Features Guiding Management
- Number and size of cystic components: Risk of malignancy increases when size ≥ 3 cm
- Septations and solid components: Mural nodule has a 87% Sp and 56% Sn for malignancy
- Main pancreatic duct (MPD) dilatation and communication with the cystic lesion: MPD > 10 mm has a 77% Sp and 67-92% Sn for malignancy
Sendai Criteria
High Risk Stigmata- Jaundice
- MPD ≥ 10 mm
- Enhancing solid component
- Size ≥ 3 cm
- MPD 5-9 mm
- Non-enhancing mural nodules
- Thick enhancing cystic wall
- Lymphadenopathy
- Abrupt Duct Termination
Management
- Any worrisome features present = Endoscopic Ultrasound (EUS) and Cyst aspiration with fluid analysis
- Any high risk stigmata present or suspicious cytology on EUS = Surgical resection
- MCN or SPN = Surgical resection
- Serous cystadenoma
- 2-3 cm: F/U every 2 years
- ≥ 4 cm: consider resection
- IPMN:
- Main duct and combined type: Surgical resection (but depends on location, pt. age/clinical status)
- Branch duct type = follow if < 3 cm and contains no solid components
- If < 2cm F/U q1yr; if growth FU q6mo
- If 2-3 cm F/U q6mo x 2 years, then q1yr
- Consider EUS (if mucinous then resect)
- If growth ≥ 3 cm, resect
What the Clinician/Surgeon wants to know
- Number of cystic lesions
- Largest cystic lesion
- Unilocular
- Multilocular: Microcystic (<2cm) or Macrocystic (> 2cm)
- Lesion size
- Lesion location: Head/Body/Tail
- Septations: None/Thin/Thick (> 2mm)
- Solid components: Present/Absent
- Calcifications: None/Coarse/Rim/Central
- Communication with MPD: Present/Absent
- Main pancreatic duct diameter: > 5 mm/Not dilated
References
- Gandhi NS and Hecht E. “Adding Value to Clinical Care: Structured Reporting of Pancreatic Pathology”, Workshop Session Presentation, Society of Abdominal Radiology Annual Meeting, 2015
- Tanaka M,Fernández-del Castillo C, Adsay V,et al. International consensus guidelines 2012 for the management of IPMN andMCN of the pancreas. Pancreatology(2012) May-Jun;12(3):183-97
- DewhurstCE, Mortele KJ. Cystic tumors of the pancreas: Imaging and management. RadiolClin N Am 50(2012)467-86
- SahaniDV, Miller JC, and Fernandez C et al. Cystic pancreatic lesions: Classificationand Management. ACR (2009)
- SahaniDV, Kadavigere R, and Saoker A. Cystic Pancreatic Lesions: A SimpleImaging-based Classification System for Guiding Management. Radiographics (2005)25:1471-1484