That is, what do you do if your neck or c-spine CT scan is obtained with the head turned, and you see what looks like a Type I rotatory atlatoaxial subluxation?
The patient below was referred to our institution in a cervical collar for management of atlantoaxial rotatory subluxation.

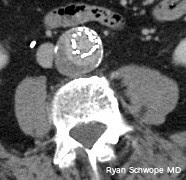
30-year-old man: Thick reconstructions centered at C1-C2 from a CTA obtained with the head rotated to the left. There is apparent type I atlantoaxial rotatory subluxation.
Let's take a look at what we know:
- Normal maximum rotation of the head on body is between 60–80°.
- Normal maximum rotation of C1 on C2 makes up for about half of that: 30–45° (although some sources say it can be up to 50°).
- Cutoff for abnormal C1-C2 rotation: >45° or >56°, depending on source.
One study that was helpful came from the forensic field. Pathologists had noted that CTs of cadavers, which are impossible to position "correctly," were resulting in a lot of over-calling of atlantoaxial rotatory subluxation. When they looked at their data they found:
- 19 cases where the C-spine was stable on autopsy. In those 19, 13 had suspected rotatory subluxation based on on CT (false positives)
- The C1-C2 angle in those 13 false positives were between 16–47°.
- All false positives were type I.
- 2 cases where the C-spine was unstable at autopsy. In those 2 cases, 1 had suspected rotatory subluxation on CT (true positive).
- The C1-C2 angle in the 1 true positive was 42°.
- There was a significant association between the false positives and degree of head rotation.
Generalizing this cadaveric study suggests that we need to be careful in calling type I atlantoaxial rotatory subluxation in asymptomatic patients who simply happen to have their head turned in the scanner. This supports the conclusions from a study in living, asymptomatic patients, where the authors showed that incomplete rotational facet displacement on CT was not sufficient to define subluxation.
References
- Fielding JW, Hawkins RJ. Atlanto-axial rotatory fixation. (Fixed rotatory subluxation of the atlanto-axial joint). J Bone Joint Surg Am. 1977 Jan;59(1):37-44.
- Persson A, Falk J, Berge J, Jackowski C. Atlanto-axial rotatory subluxations in postmortem CT: radiologists be aware of a common pitfall. Forensic Sci Int. 2013 Feb 10;225(1-3):9-14.
- Mönckeberg JE1, Tomé CV, Matías A, Alonso A, Vásquez J, Zubieta JL. CT scan study of atlantoaxial rotatory mobility in asymptomatic adult subjects: a basis for better understanding C1-C2 rotatory fixation and subluxation. Spine (Phila Pa 1976). 2009 May 20;34(12):1292-5.